Abstract
Patients with secondary AML or MDS derived AML have poor outcomes compared to de-novo AML. The benefits of intensive chemotherapy without anticipated transplant consolidation have been previously doubted. Outcomes in USA trial centres have not often been closely replicable in real world settings. From November 2018 CPX-351 has been available in the UK for secondary AML, therapy related AML, AML with MDS related Karyotype (AML-MRC) and licensed but not funded for AML with myelodysplastic related changes.
Objectives
Here we report our experience specifically on patient outcomes and toxicity across 5 Hospitals in West Midlands, UK
Methods
Patients receiving CPX 351 outcomes were evaluated retrospectively from 2018 to 2021. Baseline genetics, CPX 351 indications, patient's comorbidities, overall survival, remission status, number of cycles delivered, early mortality, reasons for early discontinuation, intensive care admission and time for neutrophil recovery (>0.5) was recorded. Time-to-event outcomes reported here are from a data cut on 01-06-21
Results
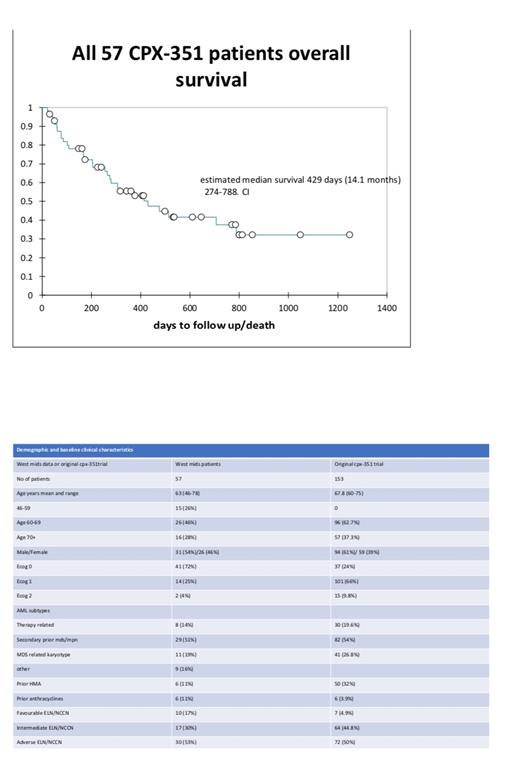
In a total cohort of 57 patients baseline characteristics are shown on table 1 and compared with the original trial CPX-351 group. Median follow up was 376 days (range 21 to 1248 days).
The mean age was 63, 17 patients were under 60, 31 males and 26 females.
The most common indication for CPX-351 was AML with antecedent MDS/MPN 51% (N=29), therapy related 14% (N=8), MDS related karyotype (AML-MRC) 19% (N=11) and 16% (N=9) other patients.
Mean Charleston co-morbidity score was 2.7 (range 0-6), 10.5% (N=6) had previous non myeloid malignancies, 8.7% (N=5) had prior ischaemic heart disease, only 3.5% (N=2) had ejection fractions under 50%.
The most common mutations were TP53 21% (N=12), ASXL1 15.7% (N=9), TET2 15.7% (N=9), IDH2 10.5% (N=6), RUNX1 10.5% (N=6), SRSF2 7% (N=4), JAK2 3.5% (N=2), FLT3 5% (N=3), NPM1 5%(N=3) and IDH1 5% (N=3).
MRC cytogenetic risk was adverse in 19 patients (33%), intermediate in 35 patients (61%) and favourable in 3 patients (5%).
30 patients (53%) had adverse European Leukaemia Network classification, 17 (30%) had intermediate and 10 (17%) had favourable.
30-day mortality was 3/57 (5%), 60-day mortality was 6 (10.5%) comparable to the 5.9% and 10.6% rates for the original trial. 9% or 5/57 patients were admitted to ITU with 2 survivors beyond 60 days. Neutropenic fever requiring antibiotics was 100% whereas only 5/57 (9%) had radiological evidence of fungal infection. Only one patient died from COVID 19.
The mean time to neutrophil recovery was 35 days with a range of 12 to 84 days.
29 patients completed 1 cycle, 25 completed 2 cycles, only 3 completed 3 cycles. The reasons for stopping were death, refractory disease, drop in performance status, alternative chemotherapy chosen or moving to transplantation (39%).
Composite remission rate including CRi was 61% 36/57, adverse ELN group demonstrated 50% 15/30, intermediate 76% 13/17 and favourable 80% 8/10. Mutated P53 was associated with a 50% 6/12 rate whereas in wild type P53 the remission rate was 60% 30/45.
Overall median survival from diagnosis was 429 days [95% CI 274 to 788 days]. To compare with the original trial, we removed the under 60s and those with less than 1 year follow up, in this cohort of 30 patients the median survival was 289 days (9.5 months) with 95% CI of 255 to 476 days.
P53 mutated patients had an estimated median survival of 257 days versus wild type p53 with 524 days hazard ratio of 2.418 (CI 1.077 to 5.248) with p value of 0.032. Median survival for ELN groups was 373 days (adverse), 413 days (intermediate) and not reached for favourable.
Of the 36 patients who achieved a remission, 22 went on to receive an allogenic transplant with follow from 254 to 1248 days, median survival estimated 706 days (95% CI 429-not reached). Patients in remission who haven't received a transplant have a similar estimated survival of 788 days (305-not reached) pending longer follow up.
Conclusion
This is the first UK multicentre analysis to show comparable results to the landmark trial (median survival 9.5 months in equivalent cases). The improved overall remission rate 61% versus the 47% in the trial and the longer median survival 14 months versus 9.5 months in the trial is expected given the younger age and increase in favourable risk genetics. This study therefore supplies further data of CPX-351 efficacy in younger patients not included in the original studies and may now be used as a standard comparator arm.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal